The Science Behind Champagne (Or Sparkling Wine)
Nov 13, 2023

The elegance of champagne’s bubbles offers a unique experience both in aesthetics and taste. There’s a reason this special beverage is almost synonymous with celebrations. While there’s no doubt that champagne is unique, have you ever wondered what makes it so? Keep reading to learn the science behind champagne and sparkling wine.
What Is the Difference Between Champagne and Sparkling Wine?
Champagne is a type of sparkling wine. However, only sparkling wine produced within the Champagne region of France can be called champagne. Sparkling wine from anywhere else isn’t eligible to be named after the famed wine region. Champagne is often considered to be more luxurious and of higher quality than other sparkling wines but excellent sparkling wines are produced all over the world.
How Is Champagne Produced?
To understand all that happens within your tall glass of champagne, it’s important to know how that bubbly substance is produced. The production of the champagne is where the magic begins.
The Fermentation Process
Fermentation is the chemical breakdown of a substance by bacteria, yeasts, and other microorganisms and typically involves effervescence (the bubbles) and the giving off of heat. A lot of alcohol goes through this process. This process allows the yeast to dissolve the glucose and fructose in the grape juice and convert them into carbon dioxide and ethanol.
Champagne’s Second Fermentation
What makes champagne unique from other alcoholic beverages is the second fermentation it undergoes. This fermentation is within the bottle to trap the carbon dioxide, which dissolves into the wine to form the bubbles we’ve come to love.
How Does Henry’s Law Affect Champagne?
The bubbles in champagne and sparkling wine are caused by the carbon dioxide that has dissolved into the liquid. Henry’s Law states that the pressure of the gas above a solution is proportional to the concentration of the gas in the solution. This means that the higher the pressure from the gas above the champagne, the more carbon dioxide dissolves into it. In an unopened bottle of champagne, the carbon dioxide dissolves into the wine and is balanced with the gas in the space between the cork and the liquid.
The Carbon Dioxide in Champagne Bubbles
When you uncork the bottle of champagne, the gas is released and throws off that balance. The carbon dioxide leaves the champagne via the bubbles to reestablish the balance. This is what causes the bubbles to come rushing out of the bottle when you open it.
What Makes Champagne So Unique?
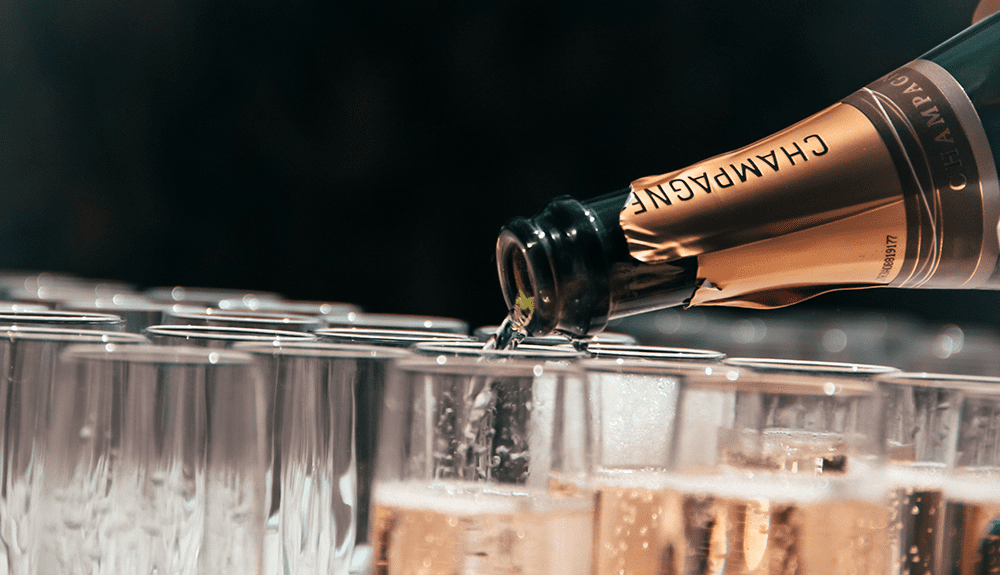
There are more than 600 different chemical compounds within the carbon dioxide and champagne, lending to the unique flavors and aromas. However, even with the explosions of flavors, champagne would only be white wine without the appearance of the bubbles. They’re more than aesthetically pleasing and fun to watch.
The Tastiness of the Champagne Bubbles
As those bubbles ascend to the top of the glass, they bring molecules of flavor and aroma up with them, which then explode on the surface. These mini-explosions stimulate the senses of smell and taste, which makes drinking it much more enjoyable. It’s this unique taste that makes champagne the ideal companion for these delectable Christner’s dishes.
Order Champagne With Your Next Christner’s Meal
After this crash course, we know you’ll be thirsty for a glass of champagne. Thankfully, Christner’s has got you covered. With our extensive list of wines, you can believe that we have our fair share of delectable champagnes. Enjoy a cool glass with us or invite some friends and family and share a whole bottle. For recommendations, ask your server or our knowledgeable in-house sommelier.
For informational purposes only.


